PHOSPHORIC ACID
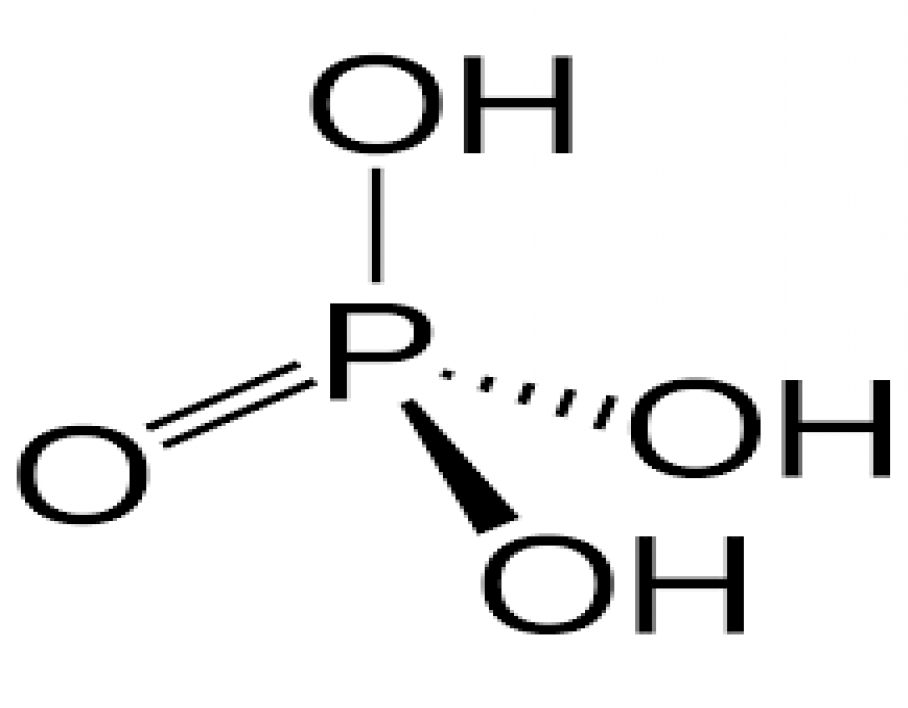
Phosphoric Acid is a colorless, odorless phosphorus containing inorganic acid.
Phosphoric acid is a sequestering agent which binds many divalent cations, including Fe++, Cu++, Ca++, and Mg++. Phosphoric acid is used in dentistry and orthodontics as an etching solution, to clean and roughen the surfaces of teeth where dental appliances or fillings will be placed. In addition,
phosphoric acid is a constituent in bone and teeth, and plays a role in many metabolic processes. Phosphoric acid appears as a clear colorless liquid or transparent crystalline solid.
The pure solid melts at 42.35°C and has a density of 1.834 g / cm3. Liquid is usually an 85% aqueous solution. Shipped as both a solid and liquid. Corrosive to metals and tissue. Used in making fertilizers and detergents and in food processing. Phosphoric acid is a phosphorus oxoacid that consists of one oxo and three hydroxy groups joined covalently to a central phosphorus atom. It has a role as a solvent, a human metabolite, an algal metabolite and a fertilizer. It is a conjugate acid of a dihydrogenphosphate and a phosphate ion.
SPECIFICATION
Molecular Formula: H3PO4 or H3O4P
Synonyms: Phosphoric acid
CAS#: 7664-38-2
Molecular Weight 97.995
Specifications Phosphoric Acid Industrial Grade Phosphoric Acid Food Grade
Appearance Colorless, transparent syrupy liquid or in very light color
Colour ≤ 30 ≤ 20
Assay (as H3PO4 ) ≥ 85.0% ≥ 85.0%
Chloride(as Cl- ) ≤ 0.0005% ≤ 0.0005%
Sulphats(asSO42- ) ≤ 0.005% ≤ 0.003%
Iron (Fe) ≤ 0.002% ≤ 0.001%
Arsenic (As) ≤ 0.005% ≤ 0.0001%
Heavy metals,as Pb ≤ 0.001% ≤ 0.001%
Oxidable matter (asH3PO4) ≤ 0.012% no
Fluoride,as F ≤ 0.001% no.

